The combination of two technologies—induced pluripotent stem cells and high-density microelectrode arrays—offers an unprecedented platform to study human brain activity in detail outside the body.


Image credits: M. Oeggerli Copyright Advanced Biology, Wiley Online Library.
Back in 2006, Prof. Yamanaka and colleagues revealed a technique to reprogram adult skin cells into embryonic-like cells called induced pluripotent stem cells or iPSCs. This Nobel Prize winning technology enables access to human neurons derived from adult blood or skin cells in a high-throughput manner, thus opening up the possibilities to study neurological disease mechanisms and to advance drug discovery. Being able to capture and analyze the activity of human iPSC neurons is key to understand the neural processes indicating disease, as well as the effect of compounds. Silvia Ronchi and colleagues, co-supervised by Dr. Michele Fiscella, VP Scientific Affairs of MaxWell Biosystem, and Prof. Andreas Hierlemann of the Bio Engineering Laboratory at ETH Zurich, recently reported in Advanced Biology a platform to obtain reproducible and detailed neuronal activity readouts at the network, single-neuron, and subcellular scales. Complementary-metal-oxide-semiconductor (CMOS)-based high-density microelectrode arrays (HD-MEAs, MaxOne System by MaxWell Biosystems) were extensively used for phenotypic functional characterization of iPSC-derived disease models for Parkinson’s disease (PD) and amyotrophic lateral sclerosis (ALS).
Human iPSC-derived neurons show unique activity patterns across development
In this work, the following human iPSC-derived neuronal cell lines were recorded and analyzed: (1) Healthy human dopaminergic neurons and (2) human dopaminergic neurons modeling PD via A53T a-synuclein mutation; and (3) healthy human motor neurons as well as (4) human motor neurons modeling ALS via TDP-43 Q331K mutation. Spontaneous extracellular action potentials were detected from all samples and were tracked every week from days in vitro (DIV) 7 to 21. To extract multi-scale datasets, the following neuronal assays were performed:
- Activity Scan – an overview of the activity of the whole neuronal population per well, providing metrics such as mean firing rate, mean spike amplitude, mean inter-spike interval coefficient of variation, and percentage of active electrodes;
- Network Recording – a means to characterize the synchronicity and oscillatory activity of the neuronal network, providing burst metrics such as burst duration, mean inter-burst interval, burst per minute, and burst shape;
- Axon Tracking – a method to extract subcellular features of the neuronal activity, including action potential propagation velocity.
Among the comprehensive metrics obtained, burst shape and axonal conduction velocity provided distinctive phenotypes between healthy and diseased neurons, as well as between different cell types.
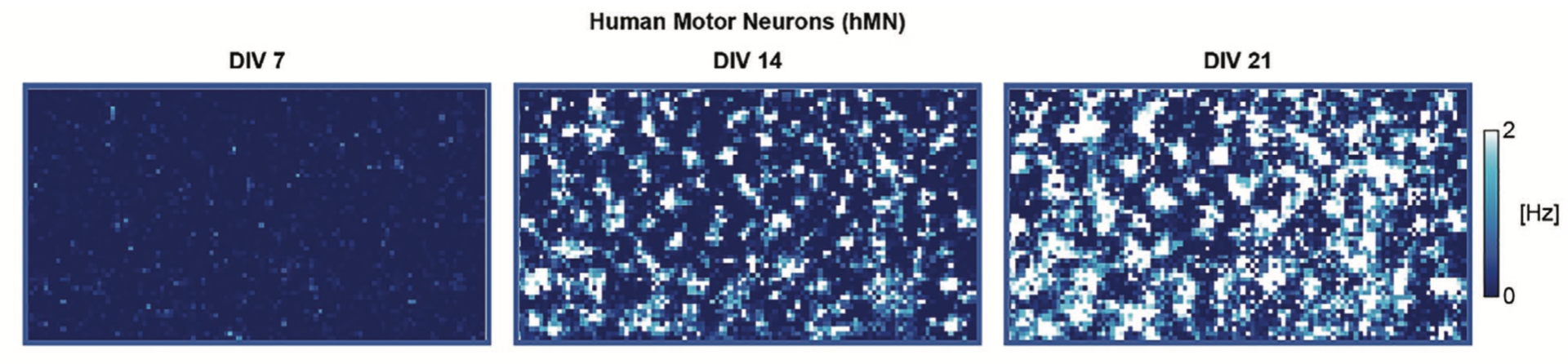

Firing rate activity maps of the same human iPSC-derived motor neuron culture across multiple days.
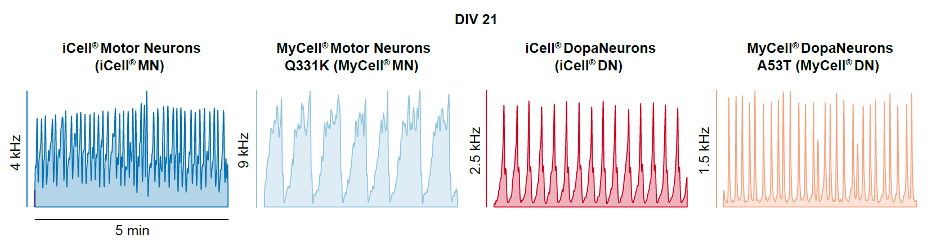

Oscillatory patterns and burst shapes observed across different neuronal cell lines.
Retigabine diminishes spontaneous activity in motor neurons
The authors performed a proof-of-concept study on the reproducibility of the functional readouts from many samples using the combined h-iPSC and HD-MEA platform for drug application. Retigabine, an anti-convulsant drug used for epilepsy treatment, has been applied to motor neurons, expecting a reduction in spontaneous bursting activity based on published literature. The results were remarkable. Small drug dosage changes were detected in HD-MEA recordings. More importantly, the well-to-well variability in HD-MEAs for the same condition is low, thanks to the statistical power per well brought by the capability to record thousands of neurons at the same time. The “secret” is being able to focus recordings to where the neurons are, such that all channels are recording meaningful data—which is possible using MaxOne’s Network Assay.
Opportunities towards the development of drugs for neurological diseases
Being able to reliably discriminate neuronal activity between healthy and diseased iPSC lines, measured across multiple scales in vitro, is a first big step towards an approach to understand and predict human brain functions that lead to disease. Moreover, an in vitro platform achieved by combining iPSC and HD-MEA technologies can be scaled-up for screening hundreds to thousands of compounds for neuronal function, which is currently lacking in the drug development pipeline. Reproducible electrophysiological metrics acquired in an easy manner will definitely empower scientists to gain speed and reliability in phenotyping cell lines and drug effects, therefore facilitating the development of cures for brain diseases.
We at MaxWell Biosystems congratulate Silvia for this significant work, which serves as a foundation for future iPSC and HD-MEA platform applications, and for her successful PhD defense.
Citation
S. Ronchi, A. P. Buccino, G. Prack, S. S. Kumar, M. Schröter, M. Fiscella, A. Hierlemann,
“Electrophysiological Phenotype Characterization of Human iPSC‐Derived Neuronal Cell Lines by Means of High‐Density Microelectrode Arrays“, Advanced Biology (2021) Vol. 5:3 2000223. (DOI: 10.1002/adbi.202000223)
All images are c/o Silvia Ronchi and copyright by Advanced Biology, Wiley Online Library.
 English
English


