Maria Sundberg from the Boston Children’s Hospital, Harvard Medical School, and colleagues, recently reported in Nature Communications a study on the reciprocal copy number variations (CNVs) of 16p11.2 gene region, associated with a wide spectrum of neuropsychiatric and neurodevelopmental disorders. For the functional analysis of the developing iPSC-derived dopaminergic (DA) neuron network, complementary-metal-oxide-semiconductor (CMOS)-based high-density microelectrode arrays (HD-MEAs), MaxOne System by MaxWell Biosystems was extensively used.
      | "We have studied the role of 16p11.2 copy number variations in dopaminergic neuron differentiation and function in vitro. This project is important because it helps to identify different neuronal dysfunctions in human cells with genetic mutations that are associated with neurodevelopmental and neuropsychiatric disorders. This project also facilitates identification of novel druggable targets for development of new treatments for these disorders in the future. During this work we established a collaboration between different laboratories, which made this a great project to work on and allowed us to learn different techniques from different groups. I think that combining the strengths of different research labs made the studies and data analysis efficient." - Dr. Maria Sundberg |
16p.11.2 Reciprocal copy number variations (CNVs) in neuropsychiatric disorders
16p.11.2 CNVs have been implicated in childhood-onset developmental and neuropsychiatric disorders, such as schizophrenia, bipolar disorder, and autism. This region contains 29 coding genes, most of them expressed in the brain. Autism is more common in patients with deletion of this gene region and schizophrenia and bipolar disorders are strongly associated in the duplication. Moreover, previous brain imaging studies revealed that patients with deletion of this gene region show increased brain volume, and the opposite phenotype has been detected in patients with duplication of this gene region.
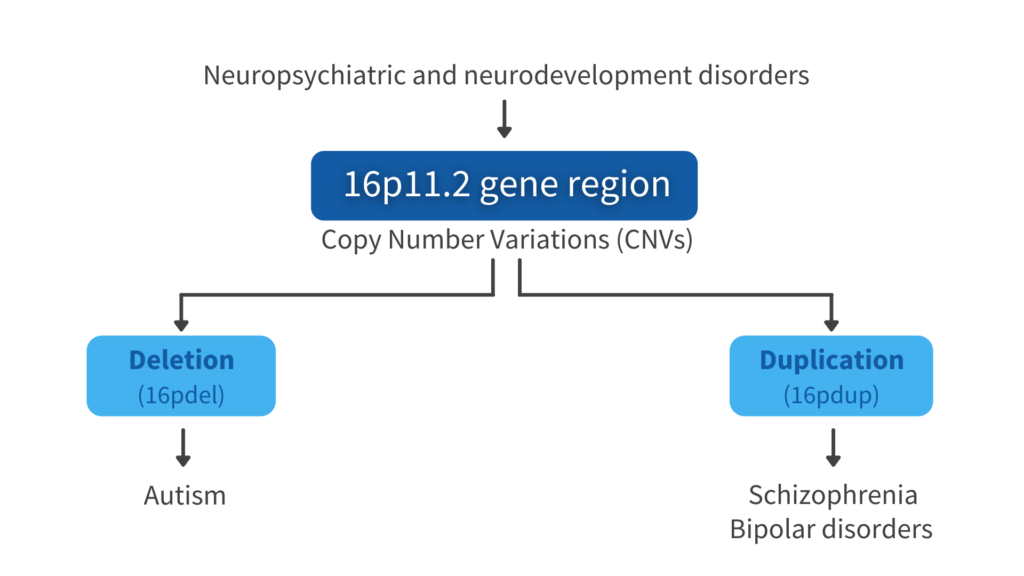

Neuropsychiatric and neurodevelopmental disorders
Dopaminergic neurons in neuropsychiatric disorders

Human iPSC derived dopaminergic neurons. Copyright by Sundberg, M .et al, Nat Commun 12, 2897 (2021). https://doi.org/10.1038/s41467-021-23113-z
Dopaminergic neurons are a specific cell type present in the brain and dopaminergic neural dysfunctions have been implicated in autism and schizophrenia. Especially in autistic patients, antipsychotic medications, like the dopamine receptor blockers haloperidol and risperidone, have been used to treat irritability, anger, and communication deficits. These same medications have been used to treat psychotic behaviors in schizophrenia.
This study brings novel insights in new molecular targets for drug development for these neuropsychiatric disorders from the characterization of DA neurons that carry these copy number variations.
From iPS cells to drug screening
The first step of the study consisted in creating isogenic human iPSC cells previously edited with CRISPR Cas9 method to contain either16p11.2 deletion (16pdel) or duplication (16pdup). Then, iPSC cultures were differentiated into the DA neurons, using a previously established protocol with minor modifications. The neural cells and their respective disease phenotype were characterized by looking at their differentiation capacity, electrophysiological properties, and transcriptional gene expression profiling. Lastly, drug screening was performed to identify molecular targets for patient treatment. In this featured article, we will focus more on the functional phenotyping of DA neurons.
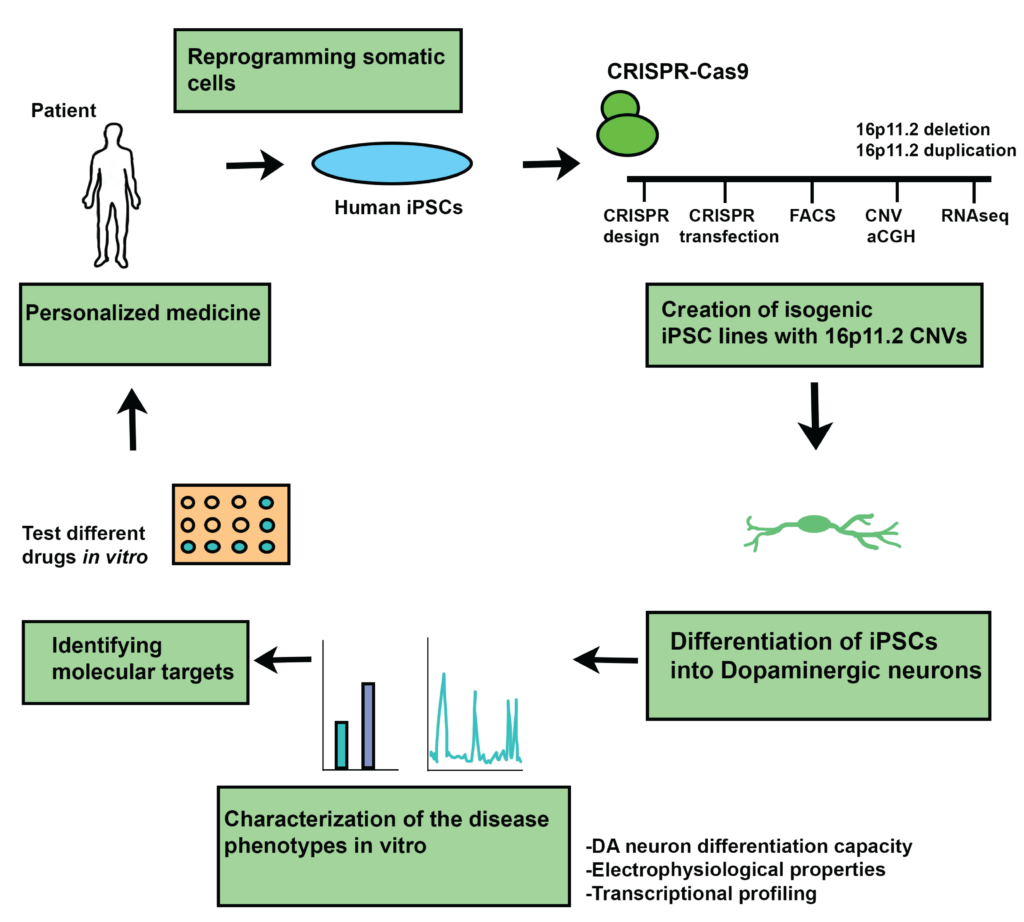

Figure prepared by Dr. Maria Sundberg
      | "In general, it was challenging to differentiate and characterize the dopaminergic neuron cell populations from human pluripotent stem cells. This included differentiation of iPSCs into neurons, sorting them with FACS, and optimizing the co-culture conditions with human astrocytes for the functional assays. Also, cell culture media formulation and MEA chip coating took few rounds of testing before we got them to work optimally." - Dr. Maria Sundberg |
Functional characterization of dopaminergic neuron networks activity through electrophysiology
Focusing on single cell characterization, the authors performed patch-clamp experiments to measure spontaneous activity and excitability of neurons. The method also allowed to inject Alexa fluor dye to image and characterize the morphological properties of the DA neurons. The results showed increased excitability in the 16pdel neurons, as well as significantly larger soma size. No significant different s were found in the number and length of neurites.
To look more in detail to the network development of the DA neurons in vitro, two different methods were used: low-density microelectrode array (MEA), and high-density MEA using the MaxOne System by MaxWell Biosystems.
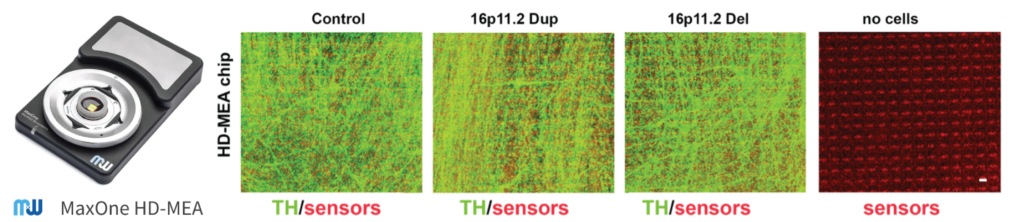

Figure adapted from Sundberg, M .et al, Nat Commun 12, 2897 (2021). https://doi.org/10.1038/s41467-021-23113-z
“The advantage of the HD-MEA platform is that it can record extracellular network function simultaneously from 1024 sensors selected out of 26,400 sensors per chip yielding very high-resolution data of the neuronal activity during the formation of the network connections” stated the authors in the publication.
In these two methods, the chips were coated with polyethilenime (PEI) 0.1% and laminin. 125 000 sorted neurons were cultured on top of the chips together with 8 000 commercially available human astrocytes. The activity of the developing networks was measured over four weeks, in parallel from both platforms.
      | "With the MaxOne platform we were able to identify the dysfunction in the patient specific neuronal networks, which was the crux of this work. To do this we analyzed multiple features from the neuronal networks, including the frequency of network activity, network synchronization, and the bursting phenotypes of the dopaminergic neurons." - Dr. Maria Sundberg |
16pdel dopaminergic neuronal networks have increased network activity
Using MaxOne, it was possible to identify the location of active neurons on the chip, generate an activity map per culture per time point, and extract features such as the firing rate and spike amplitude. In this work, it was shown that the 16pdel neurons have significantly higher frequency of neuronal spiking activity compared to control and 16pdup networks.
Additionally, MaxOne was used to record continuously from the active neurons to investigate synchronized neuronal firing or network burst, which is indicative of network formation. With the Network Assay, one can identify the active electrodes, where neuronal activity has been detected. It was observed that the fraction of active electrodes with synchronized activity increases over time for 16pdel networks compared to control and 16pdup.
To characterize the effects of 16p11.2 CNVs on the development of the bursting patterns in the iPSC-derived neuronal network, the number of bursts per minute and the number of spikes per burst were measured per all recording sensors in MaxOne chips. The 16pdel neurons showed significantly increased number of bursts and number of spikes per burst compared to control or 16pdup populations.
More detailed analysis of the duration of the bursts, in these neuronal networks, showed that there is a trend towards increased burst duration in the 16pdel neurons. However, there was no statistical difference between the different groups.
Time intervals between bursts was significantly decreased in the 16pdel neurons compared to control and duplication population. This data indicates that these 16pdel networks are firing action potentials more frequently compared to other populations.
Detailed burst analysis of the dopaminergic neuron networks
The next step was to do a more detailed analysis of the active channels with the highest burst rates, between these different genotypes, using MaxOne. The 200 most active channels were selected, and raster plots showed that the 16pdel networks have significantly higher burst rate. In addition, they have significantly higher number of bursts per spikes per chip compared to other populations.
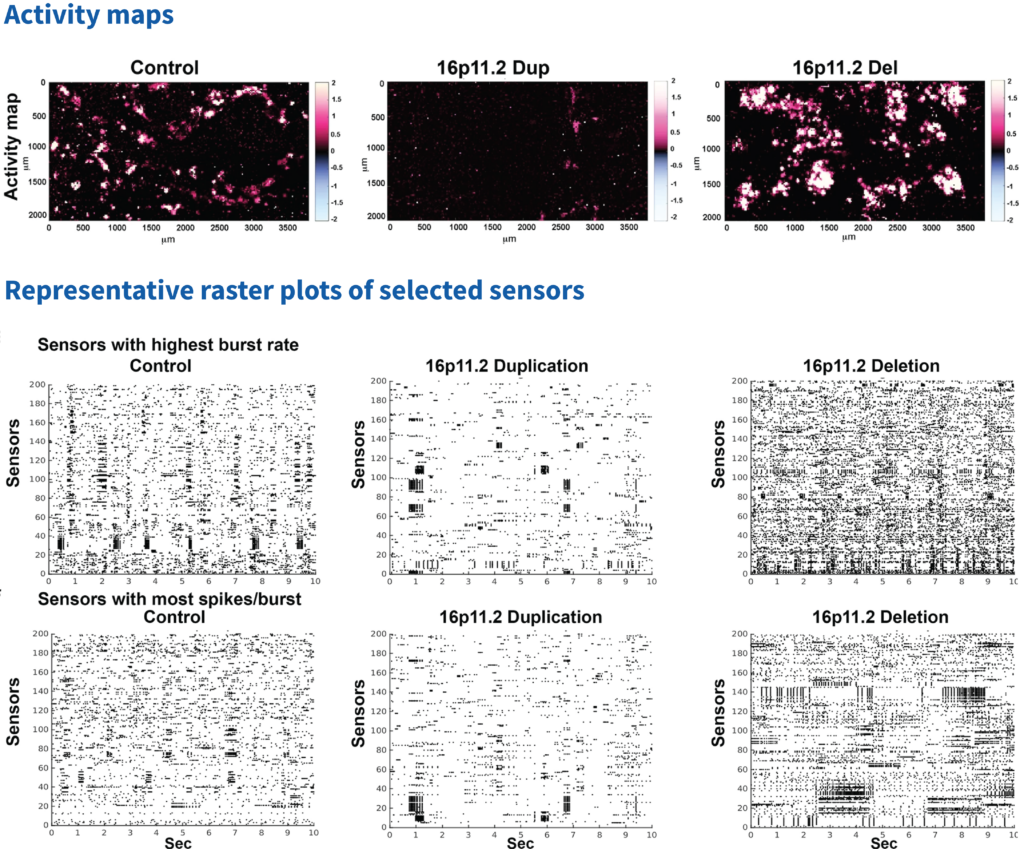

Figure adapted from Sundberg, M .et al, Nat Commun 12, 2897 (2021). https://doi.org/10.1038/s41467-021-23113-z
Understanding the molecular mechanism involved and rescue of the network activity in dopaminergic neurons with 16p11.2 deletion
To identify the molecular mechanism behind the cellular deficits in these studies, the authors focused in one out of the 29 genes coded by 16p11.2 region, the KCTD13. This gene codes for a protein that plays an important role in the functional development of cortical neurons and interacts with Cullin3-ubiquitin complex, which regulates activation of RhoA. In 16pdel population, expression of KCT13 is significantly downregulated compared to control and duplication populations, thus increasing rhoA expression.
Low density MEA measurements were performed with cells treated with Rhosin, a specific RHO-GTPase inhibitor and network activity was in accordance with HD-MEA platform: 16pdel neurons had significantly higher mean firing rates compared to control or duplication population.
     
| "In this study, we identified RhoA pathway as the potential culprit for the network dysfunctions. Its inhibitor, Rhosin, was shown to be useful in rescuing the hyperactivity of dopaminergic neuron networks carrying a 16p11.2 deletion. In the future this compound could be tested in animal models to verify its safety and efficacy. The RhoA pathway and its inhibitors may serve as a potential therapeutic target for treating patients with the neuropsychiatric disorders associated with 16p11.2 deletion." - Dr. Maria Sundberg |
Conclusion
This study provided detailed disease phenotyping in human iPSC-derived DA neurons with 16p11.2 deletion:
- 16p11.2 deletion DA neurons are hyperexcitable, have increased network activity and synchronization compared to control dopaminergic neurons.
- 16p11.2 deletion DA neurons have significantly increased bursting compared to control dopaminergic neurons or duplications neuron networks.
- Expression of RhoA was increased in the 16p11.2 deletion dopaminergic neurons.
- Rhosin treatment rescued the network activity in the deletion neurons.
      | "We were able to identify functional disease phenotypes in the human iPSC-derived dopaminergic neurons with 16p11.2 deletion. Importantly, we found that Rhosin, which specifically targets the RhoA pathway, rescued these deficits in these neurons. I am currently working on characterizing cortical neuron network development in human neuropsychiatric and neurodevelopmental disorders. In this study I am phenotyping cortical neuron network development using various methods, including high-resolution imaging, RNA sequencing, and high-density MEA. In addition to this my aim is to establish drug screening experiments with these human iPSC derived cortical neuron networks using MEA and high-content imaging platforms." - Dr. Maria Sundberg |
We at MaxWell Biosystems congratulate Maria Sundberg and authors for this significant work, which serves as a fantastic example of the HD-MEA platform applications in iPSC-derived neurons research.
We are also grateful to Maria Sundberg for sharing with us her personal insight about this work.
Citation
Sundberg, M., Pinson, H., Smith, R.S. et al. 16p11.2 deletion is associated with hyperactivation of human iPSC-derived dopaminergic neuron networks and is rescued by RHOA inhibition in vitro. Nat Commun 12, 2897 (2021). https://doi.org/10.1038/s41467-021-23113-z
 日本語
日本語


