
Method Development
Every Cell has a Story to Tell.
Let’s Discover Yours
Method development with MaxWell Biosystems’ High-Density Microelectrode Arrays (HD-MEAs) involves designing and refining experimental setups, protocols, and analysis pipelines to optimize HD-MEA use for specific biological models and research goals.
Our platforms support advanced method development through flexible electrode configurations, high-resolution signal acquisition, and intuitive, powerful software featuring Application Programming Interface (API) access and open data formats. Researchers can precisely tailor recording strategies, design custom stimulation protocols, and develop advanced analysis tools to extract novel functional metrics from rich, high-density recordings.
Method development also includes integrating HD-MEA recordings with complementary techniques such as microscopy, optogenetics, calcium imaging, and patch clamp electrophysiology. These multimodal approaches provide a more comprehensive view of neuronal structure and function, enabling cross-validation and deeper mechanistic insight. The compact design of the MaxOne recording units facilitates seamless combination with microscopy, environmental control systems, and custom lab setups. Low light sensitivity further supports integration with optogenetic tools and live-cell imaging.
Whether refining protocols for brain organoids, optimizing stimulation strategies, advancing large-scale data analysis, or developing multimodal workflows, MaxWell Biosystems’ flexible, automation-compatible HD-MEA platforms empower tailored method development that drives innovation across neuroscience, disease modeling, and drug discovery.

Combine HD-MEAs with imaging, optogenetics, and more
Achieve rich, multimodal experimental readouts by easily integrating HD-MEAs with complementary techniques such as microscopy, optogenetics, calcium imaging, and patch clamp. The compact design of the MaxOne recording unit and low light sensitivity of the HD-MEA chips ensure seamless compatibility with advanced setups.
Develop your own experimental protocol and analysis
Open data formats and built-in API support allow scientists to build tailored recording and stimulation workflows. Dedicated analysis pipelines can be developed to extract novel functional metrics tailored to specific applications.
Connect to automated lab workflows
Enable high-throughput, automated workflows by integrating the MaxTwo system with robotic handling systems, liquid dispensers, and external controllers. The built-in API ensures synchronized operation with external devices, supporting custom assays and scalable experiments.

Case studies
Insulative compression by perfluorodecalin of neuronal tissues enhances acute recordings
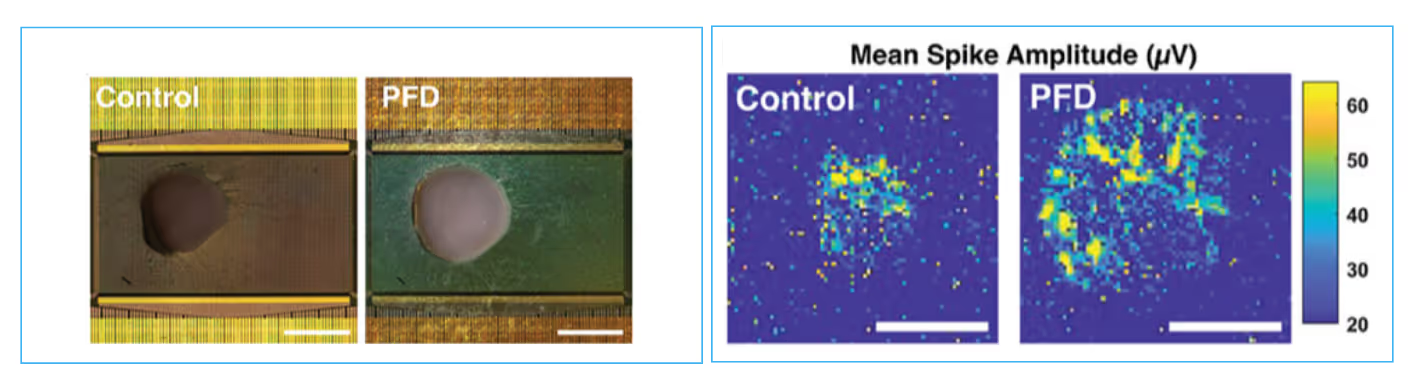
This study from the Ikeuchi lab highlights the use of perfluorodecalin (PFD) as a biocompatible liquid holder, great for acute recordings of neural tissue. The PFD film both insulates and gently compresses cerebral organoids onto the HD-MEA surface, resulting in higher spike amplitudes and detection of neural activity across more electrodes. This method also enables optimized recording quality for a wide range of samples, including organoids, cell cultures, and retinal explants. Read more in Duenki et al., Advanced Healthcare Materials, 2025.
PFD amplifies signal detection in acute organoid recordings
Image of cerebral organoid on HD-MEA before and after addition of PFD (left). Scale bar: 1 mm. Representative map of mean spike amplitude from cerebral organoids before and after the addition of PFD (right). Scalebar: 0.5 mm.
Data adapted from Duenki et al., Advanced Healthcare Materials, 2025.
Feedback-driven brain organoid platform enables automated culture maintenance and high-resolution electrophysiology
This study showcases a fully automated brain organoid platform that combines microfluidics, imaging, and HD-MEA electrophysiology for synchronized, real-time control of in vitro experiments. The system uses IoT connectivity to continuously maintain organoid health and capture high-resolution neuronal activity without manual intervention. Integrated sensors and actuators enable precise, non-invasive monitoring and data collection, streamlining workflow and advancing large-scale experimentation. Read more in Voitiuk et al., Internet of Things, 2025.
Schematic diagram of integrated IoT microfluidic chamber with automated organoid culturing and HD-MEA recordings
This automated organoid platform integrates programmable fluidics and HD-MEA recording for remote, real-time experimental control. (a) Fresh media and air are delivered by pumps and valves; (b) samples are maintained and imaged inside a sealed chamber; (c) sensors and cameras enable feedback-driven monitoring; (d) all data is processed and accessed via a web portal. (e) The system setup is shown in an incubator; (f) the compact chamber design; and (g) a cross-section detailing fluid flow and sample placement. The system supports continuous, high-resolution data collection for brain organoid cultures.
Data adapted from Voitiuk et al., Internet of Things, 2025.
Integrated multimodal workflow with simultaneous calcium imaging and HD-MEA electrophysiology
This study demonstrates synchronized dendritic spine calcium imaging and HD-MEA recordings of extracellular spiking, leveraging a flexible acquisition and analysis pipeline. By correlating fluorescence signals of presynaptic spike activity with high-resolution HD-MEA electrophysiological recordings, they were able achieve precise mapping of neuronal connectivity and function at both cellular and network levels. The platform’s open data formats and customizable workflows enable advanced, multimodal studies, supporting integration of imaging and electrophysiology for deeper mechanistic insight. Read more in Xue et al., Journal of Neural Engineering, 2022.
Experimental pipeline for simultaneous spine calcium imaging and HD-MEA recordings.
(a) Experimental setup combining upright confocal calcium (Ca2+) imaging and HD-MEA recording on live neurons. (b) Analysis pipeline for synchronizing and correlating spike and Ca2+ signals, including spike sorting and trace extraction. (c) Example region of interest containing a selected dendritic spine, with corresponding calcium trace. (d) Raster plot of spike trains and convolved traces, illustrating direct comparison of electrical and calcium activity.
Data adapted from Xue et al., Journal of Neural Engineering, 2022.
Selected Resources
A feedback-driven brain organoid platform enables automated maintenance and high-resolution neural activity monitoring

Insulative Compression of Neuronal Tissues on Microelectrode Arrays by Perfluorodecalin Enhances Electrophysiological Measurements

Revealing single-neuron and network-activity interaction by combining high-density microelectrode array and optogenetics

Acute Brain Organoid Plating Protocol with Liquid Holder
Acquire functional HD-MEA recordings from your brain organoids in no time with this unique and easy-to-use protocol.




.avif)


