Others
Every Cell has a Story to Tell.
Let’s Discover Yours
Electrically active cells play critical roles across many biological systems. Cardiomyocytes and muscle cells, for example, offer powerful in vitro models for investigating excitability, contraction, and tissue-level coordination. Our High-Density Microelectrode Array (HD-MEA) technology captures rich electrophysiological signals at subcellular, single-cell, and network levels, providing precise, label-free insights with unmatched spatial and temporal resolution.

Unbounded possibilities for your model
Adapt our system effortlessly to your biological sample. Capture reproducible, high-quality functional data regardless of your model’s complexity.
Every cell has a story to tell
Explore the behavior of electrically active cells with unmatched single-cell resolution, recording signals from many cells simultaneously to uncover intricate functional profiles.
Always at the right spot
Measure with precision. Record high-quality data with thousands of electrodes per well, perfectly positioned below your cells, ensuring reliable recordings exactly where it matters.
Don’t miss any action potentials
Acquire consistent, high-fidelity action potentials, from the largest to the smallest spikes, and fully characterize both individual cell behavior and population-level dynamics.
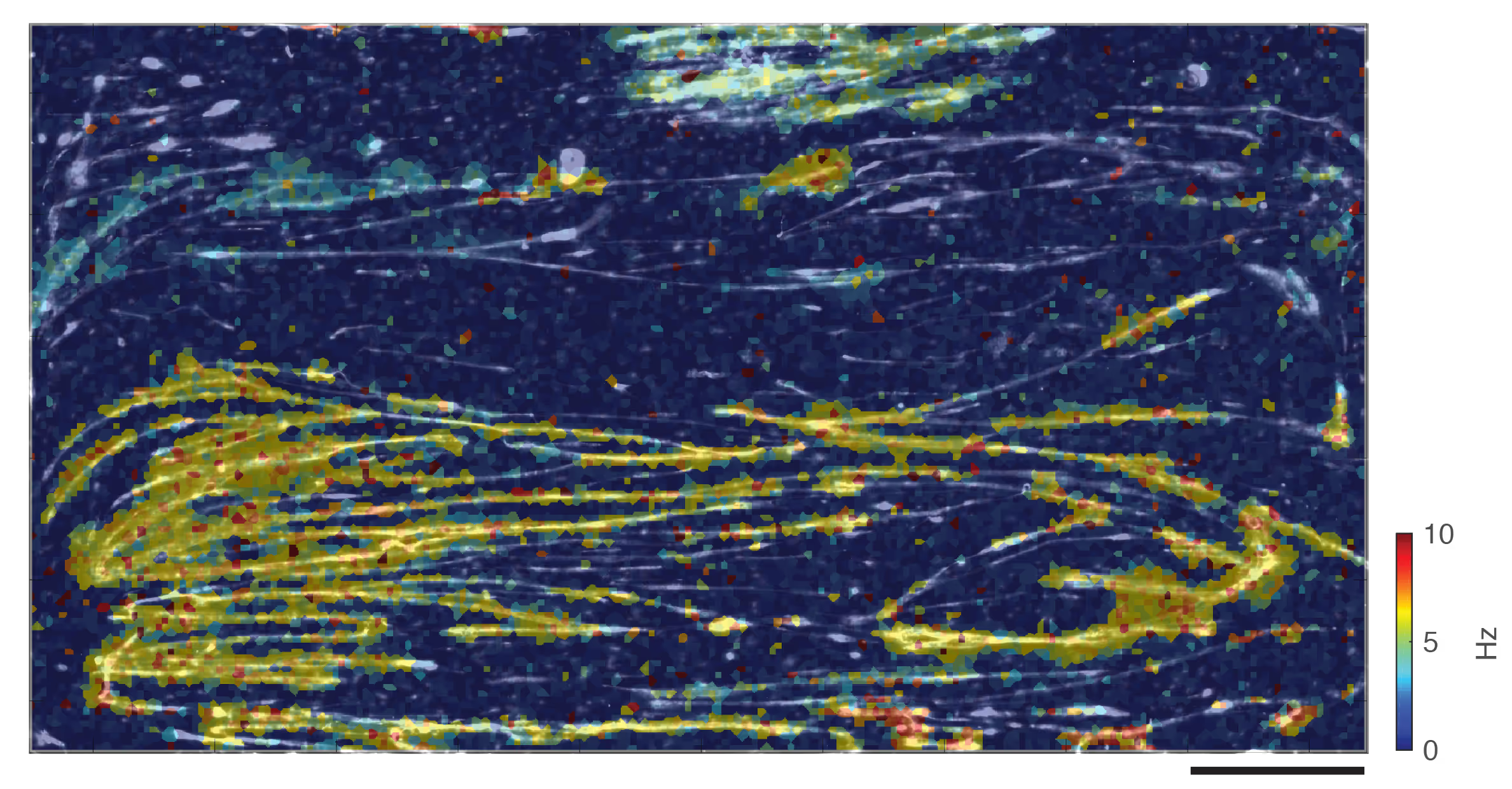
Figure adapted from Lewandowska et al., (2018), Frontiers in Physiology, licensed under CC-BY 4.0.
Functional characterization of various sample types
The MaxOne Single-Well and MaxTwo Multi-Well HD-MEA Systems enable high-resolution functional recordings from electrically active cells including muscle and cardiac models. They allow you to precisely monitor signal propagation, contraction patterns, network synchrony, and other functional features with 26,400 flexibly addressable electrodes per well.
Case studies
Analyzing burst propagation in cardiomyocyte networks
Cardiomyocyte cultures form spontaneously active, electrically coupled networks that generate rhythmic bursts of activity. These cultures can be monitored with our HD-MEA technology to visualize how bursts initiate and propagate across the culture in real time, providing insight into synchronization, conduction properties, and functional connectivity in cardiac tissue. These insights are essential for understanding functional maturation, connectivity, and arrhythmogenic behaviors in cardiac models.
Burst propagation in a primary cardiomyocyte culture recorded with HD-MEA technology.
The video shows a time-lapse of a spontaneous burst traveling across the electrode array. The activation of single cells is color-coded according to signal amplitude, revealing the direction and speed of activation.
Data courtesy of MaxWell Biosystems’ user.
Tracking electrical maturation in skeletal muscle cultures
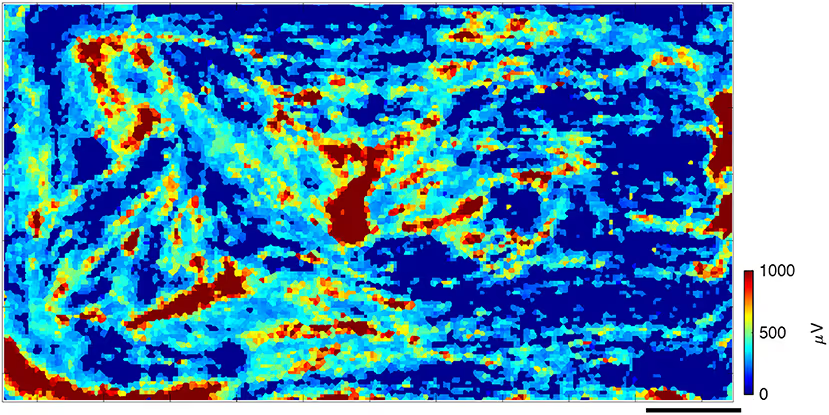
Investigate how skeletal muscle cells develop, synchronize, and communicate over time using our HD-MEA technology. The example below shows spontaneous spiking activity recorded at subcellular resolution after one month of culture, highlighting the emergence of organized electrical signaling as myotubes mature into functional, contracting networks.
Top: Spontaneous activity in primary skeletal muscle culture at 30 days in vitro.
Each square in the heatmap represents an individual electrode, color-coded by the average spike amplitude recorded during the session. The red regions indicate areas of high-amplitude spiking, while surrounding lower-amplitude filaments reflect active electrical connections between contracting myotubes. Blue regions denote areas with no detected spiking activity. The spatial heterogeneity and organization of signal amplitudes illustrates tissue maturation and functional compartmentalization. Scale bar: 0.5 mm.
Data adapted from Lewandowska et al., (2018), Frontiers in Physiology, licensed under CC-BY 4.0.
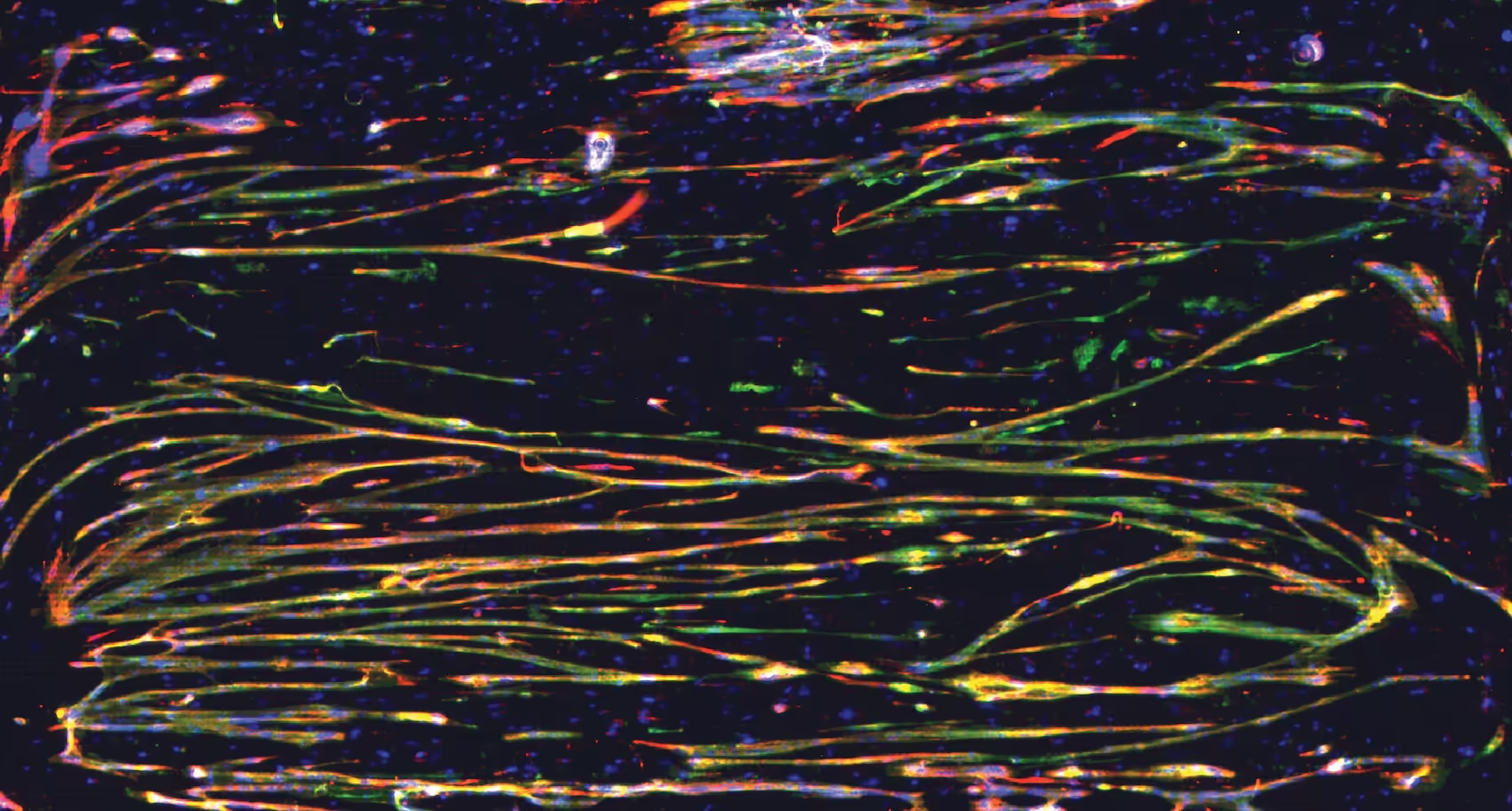
Bottom: Time- and space-resolved spiking activity in skeletal muscle culture.
(A, B) Raster plots showing 5 seconds of spontaneous activity recorded at 19 and 31 days in vitro (DIV), respectively. Each row represents an individual electrode, and each dot marks a detected spike. (C) Magnified view of the first four spikes from (B). (D, E) Spatially resolved spike timing of the second (red) and fourth (blue) spikes highlighted in (C). Scale bar in (E) is 0.5 mm and applies to (D) and (E).
Data adapted from Lewandowska et al., (2018), Frontiers in Physiology, licensed under CC-BY 4.0.
Selected Resources
Development of an innervated human skin equivalent to model nociceptive circuitry in vitro

CardioMEA: comprehensive data analysis platform for studying cardiac diseases and drug responses

CardioMEA: Comprehensive Data Analysis Platform for Studying Cardiac Diseases and Drug Responses

Repeated and On-Demand Intracellular Recordings of Cardiomyocytes Derived from Human-Induced Pluripotent Stem Cells
Long-Term High-Density Extracellular Recordings Enable Studies of Muscle Cell Physiology

MaxOne Cardiomyocyte Plating Protocol
This document describes the protocol to plate and maintain cardiomyocytes using MaxOne.