Microelectrode Arrays
Electrical recording of neuronal activity has been the preferred means of analyzing single neurons and neuronal networks (Contreras, 2004; Llinas, 1988). Electrical signals produced by neurons can be detected at a distance from the source. Several recording tools apply to different spatial scales. At the mesoscale, where local neuronal populations can be analyzed, a popular method is extracellular recording using metal electrodes. An electrode placed inside a brain slice in vitro or inserted in the brain in vivo detects electrical signals produced by the surrounding cells. A wide range of neural phenomena can be observed, from the spiking activity of individual neurons (extracellular action potentials or EAPs; bandwidth: 300-3000 Hz) to the slower network oscillations of small populations (local field potentials or LFPs; bandwidth: 1-300 Hz). Additionally, the same electrode can be used to deliver electrical stimulation to a local area in the brain. While applying this method for brain recording and stimulation is relatively straightforward, the challenge lies in the analysis of recorded data. With hundreds of possible signal sources surrounding an electrode, the specificity and selectivity of such technique is poor. Thus, extracellular recording has been widely used for analyzing population activity.
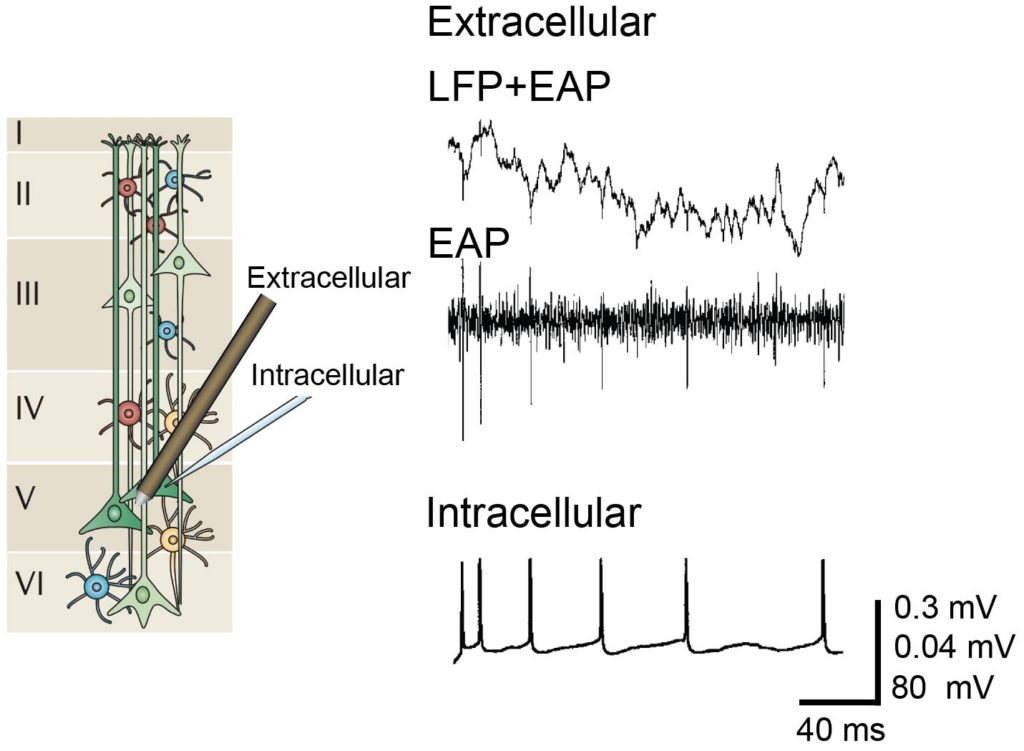

Extracellular and intracellular recording. Left: Illustration of cells across cortical layers modified with permission from (Buzsáki et al., 2012). Right: Signals of simultaneous extracellular recording and intracellular whole-cell patch-clamp recording modified with permission from (Henze et al., 2000).
To achieve high-resolution activity mapping of neuronal networks, multiple electrical sensors tightly spaced in an array can be utilized. Microelectrode arrays (MEAs, also termed multielectrode arrays) allow simultaneous long-term recording of LFPs and EAPs from a population of neurons at sub-millisecond time scale. In order to increase spatial resolution, i.e., to place thousands of electrodes per square millimeter, the area taken up by wiring between electrodes-to-readout circuitry has to be reduced. This has been made possible by using industrial complementary metal-oxide-semiconductor or CMOS technology to create high-density MEAs (HDMEAs). As an added benefit, readout circuitry, such as amplifiers and analog-to-digital converters, can be included on the same substrate as the electrodes in order to improve signal quality. The design of the on-chip signal conditioning circuitry should consider the electrode impedance and the possible sources of noise to ensure high quality signals. HDMEAs with good signal-to-noise ratio (SNR) can be used to map single neuronal activity at sub-cellular resolution and to observe network activity at the same time (Ballini et al., 2014; Frey et al., 2010).
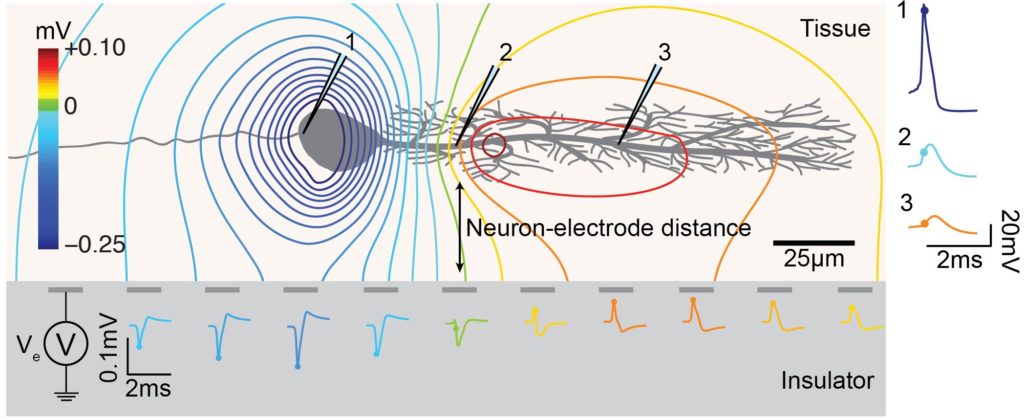

MEA neuron-electrode (fluid side). The neuron-electrode distance and neuron orientation influences the signal amplitude and shape detected at the electrodes. High spatial resolution allows for recording EAPs at several locations of a single neuron, with large negative spikes at the perisomatic area and positive spikes at the dendritic area, i.e., return current.
Switch-Matrix
The switch-matrix (SM) concept uses transistors to implement switches within the array to route signals from electrodes to readout circuitry placed outside the actual electrode array. In the SM concept, routing is operated in static mode, meaning that some electrodes are selected by opening or closing the switches and a recording is then started without changing the electrode selection. Typically, not all electrodes detect activity during an MEA experiment, thus choosing a subset of ‘interesting’ electrodes is possible. A common protocol is to first scan all the electrodes in successive recordings to determine which electrodes to later continuously record during an experiment. The advantage of this concept is, that large, low noise amplifiers can be implemented outside the actual electrode array, allowing to optimize amplifiers for best possible SNR. SM MEAs have been implemented and various degree of flexibility that the routing means provide. The availability of a large set of wires, switches, and local memory allows for even more complex routing paths that connect a subset of electrodes to the readout and stimulation channels in a flexible manner.


Passive vs Switch-Matrix MEA Architecture. Passive: Fixed wiring with electrodes directly connected to signal pads and no active circuitry. Switch-matrix (SM): Multiplexed array with flexible addressing achieved by adding more routing resources within the array.
 English
English


